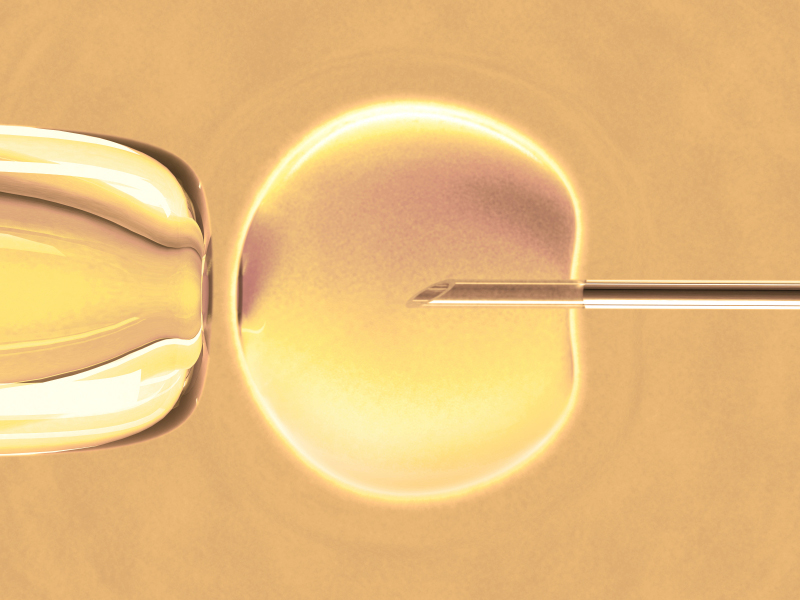
The American National Academy of Sciences has submitted its final report on “Three-Parent IVF” to the FDA[1]. These recommendations could become official US policy, depending on the next decision taken by the FDA. The expert Committee put together to write this report is of the opinion that “three-parent IVF” is “ethically acceptable” under certain conditions.
This technique involves creating embryos from 3 sources of DNA (father, mother and egg donor), to avoid the transmission of genetic diseases carried in the mother’s mitochondrial DNA. The defective mitochondria is removed from the mother’s egg and replaced by the healthy mitochondria taken from the gametes of another woman. This “medical advance” has created a stir amongst scientists specialising in reproduction (see three-parent IVF, United Kingdom). It has been authorised in the United Kingdom without any restrictions since February 2015.
The American report recommends limiting the techniques to male embryos to restrict the impact of genetic modification on future generations (boys will not transmit the modified mitochondria they receive, contrary to girls). This condition implies gender selection of embryos following in-vitro fertilisation. This “would guarantee that their grandsons will not be affected if they experience the side effects of this highly controversial IVF therapy”.
[1] Food and Drug Administration.
Nature (3/02/2016)