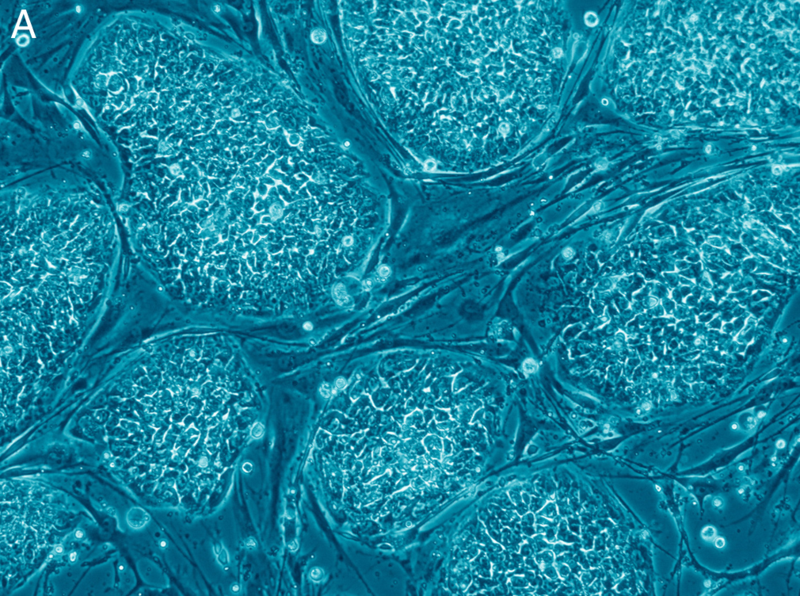
Israel’s health ministry has authorised the Hadassah University Hospital in Jerusalem to launch a phase I clinical trial using human embryo stem cells (hESC) in an attempt to treat Age-Related Macular Degeneration (ARMD).
Scientists want to implant retina cells obtained from hESC in patients suffering from ARMD in the hope of finding an effective treatment for this degenerative disease. ARMD, which is caused by the degradation of some cells in the retina (macula), currently affects 1.6 million people each yearin the United States alone.
The research team is led by Professors Benjamin Reubinoff and Eyal Banin. Both have worked on culture conditions for hESCs to differentiate them from retina cells.
This clinical trial is the “first step”, “the aim being to ensure the safety of cell transplantation and to assess the therapeutic effect”,explained Professor Reubinoff.
Note from Gènéthique:
Another American biotechnology company, Ocata Therapeutics, uses differentiated hESCs in retina cells to combat ARMD and is carrying out phase 1 trials.
The London Project to Cure Blindnessin partnership with Pfizer Laboratory is preparing the same type of trial.v
The Jerusalem post (2015/08/31)